Monoclonal Antibodies: more than 100 Years of History
History of monoclonal antibodies started with a bold prediction about “Magic Bullet” from “father of chemotherapy and immunology” Paul Erlich.
Receiving a Noble Prize for the discovery of antibodies, he postulated, that they will be soon used as tool to design a “personilized and tailored treatment from any malady”.
In 1984, 76 years later, another German researcher George Köhler toghether British-Argentinian César Milstein received a Noble Prize for discovery of a hybridoma, a first factory of monoclonal antibodies (mAbs).
What came next can be discribed as “golden rush” with dozens of mAbs hitting market every year giving thousand of patients and doctors a glimmer of hope, that advanced tumors and serious inflammatory diseases would soon be cured.
In this article you will learn what is antibody, what is the difference between monolocnal and polyclonal antibodies, how mAbs are classified and how they serve thousands of clinicians in treating cancer, autoimmune disorders, and rare diseases.
What is antibody
Immune system produce antibodies is to fight the foes of your bodies, or what your immune system recognizes as “an enemy”.
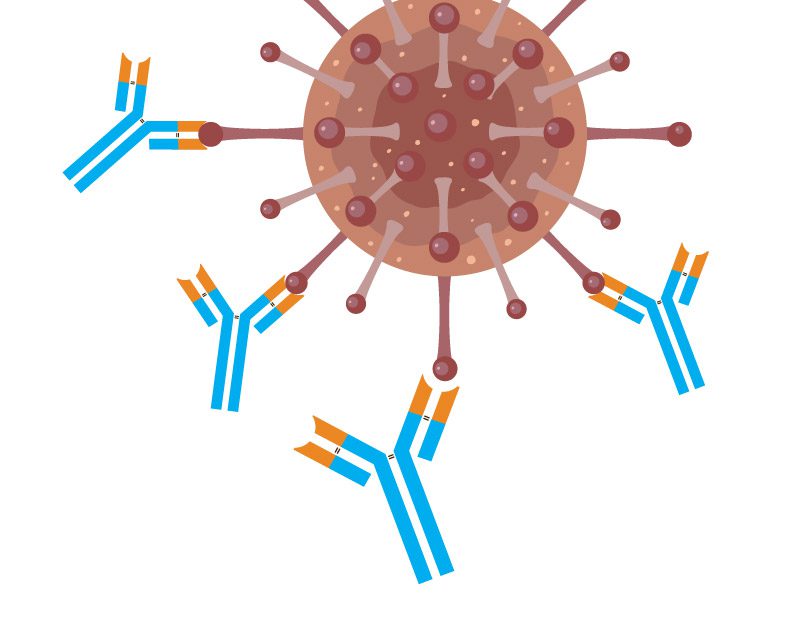
They are Y-shaped proteins, circulating in the blood or hummering around leucocytes, white blood cells, which work with them as a team to preserve integrety of your body.
Scientists discovered 5 major types of antibodies and called them immunoglobulins A, D, E, G, and M immunoglobulins (or shortly Ig). They all have different function, with dominant IgG representing 90% of antibodies circulating in the blood.
The unprecedented stability of IgG makes this class a a go to option for biotech companies for producing monoclonal antibodies.
How does an Antibody Work?
When an antibody binds a pathogen, it:
- Ensures the suspect won’t escape, by neutrilizing all its biological functions
- It calls for “reinforcement”, which might come in to ways:
- NK-killer or a phagocyte comes to devour the pathogen (scientists call this opsonization)
- Special forces called complement system can come, release enzymes that breaks integrity of the pahogen. The process is called Lysis.

How antibody became a medicine
Reaserches found a way how to grow them in lab using the “Hybridoma technique”. Hybridoma is a crossbreed of two lines of cells:
- First parent cell gives it immortality
- Second gives it values – ability to produce “pure” single line of antibodies, monoclonal, as scientists called them.
Scientists use different methods to identify, distill, and make them more stable, before it would be considered as a medicine.
Polyclonal versus Monoclonal antibodies
Monoclonal means antibodies are produced by single line (clone) cells using a single boilerplare, so all of the antibodies are identical.
Prefix poly- means many, so polyclonal antibodies are different in structure and targets.
Types of Monoclonal Antibodies
First hybridomas were grown in a mice and we fully murine. The first antibody has seen the market is Muromonab. Notice it has “mo” suffix, literally meaning “mouse” mAb.
Unfortunatelly, mouse mAbs are not ideal as a medicine. Their buildup is too “foreign”for our immune system, thus fast immune response from the body ensues the injection.
Scientists soon found a solution, a transgenic mouse, that produces three quoters of fully human antibody. Less immune reaction, more stay in the blood – more therapeutic efficacy. Infleximab and Rituximab (Rituxan) are two most famous examples of this type, aslo known as chimeric. Xi is for chimeric.
Next generation is humanized, where only six slices (CBRs) of murine antibodies left. The example is an anti-cancer antibody Trastuzumab (Herceptin) and Pembrolizumab (Keytruda). Substem -zu is for huminized.
Can you guess the next generation? If you think “human”, you are righ. A purely human antibody with minimum immunegenicity – ability to attract attention and elicit an immune response.

Full classification of mAbs
- Murine: Fully derived from mice. They can trigger strong immune responses but may be less effective in humans.
- Chimeric: one quoter – a mouse and and three quoters – a human. They are more familiar to immune our immune system, so lesser chance to develop immune response and be eliminated prematurely – before commencing good deads in the body as a medicine.
- Humanized: predominantly human, with only a small bits are from mice. There is a better complience with a human immune system and less chance to get cough and eliminated.
- Fully Human: having entirely a structure, identical to human conterpart. The risk of an immune rejection is minimal.
Monoclonal Antibody – What Happens After Injection
Mechanisms of Monoclonal antibodies do not differ from a way natural antibodies work:
- Binding. First, a monoclonal antibody binds to the specific antigen. When the antibody connects to its target, it forms a stable complex. This specificity helps ensure that the treatment focuses only on the harmful cells, limiting damage to healthy cells. This targeted binding is a critical step in the therapeutic process.
- Inactivation. Once mAb attaches to the target, the antigen becomes inactive no longer. At this step, viruses lose their ability to invade healthy cells, bacteria release their enzymes to target our tissues and organs, and some immune compounds, like interleukins, send signals promoting inflammation.
- Immune System Modulation. At this stage, the antibody calls for support, by signalling to the immune team to step in and get rid of the unwanted agent. It can go two ways:
- A phagocyte or an NK-killer will devour the antigen
- A complement system will release chemicals to break down a bug into pieces.
- Finally, antibodies can contain effects of “toxins” by signalling to the phagosome to gulp them down.
The processes described above are an oversimplification of the real-world picture. The real intricacies of the immune response and the role of antibodies are way beyond the scope of this article.
It is worth mentioning that some monoclonal antibodies are designed to ease the immune reaction and calm inflammation. Some of them might send negative signals to the “immune team” and prevent opsonisation or lysis of the cells, specifically when the immune response “goes bonkers” and attacks its normal organs.
Therapeutic Use of Antibodies
Monoclonal antibodies have many therapeutic applications. They are widely used in treating cancers, autoimmune diseases, and infections. Some common uses include:
- Cancer Treatment: mAbs can target cancer cells binding to the tumor’s key survival strategies:
- Escape from the immune system
- Aggressive reproduction
- Stealing the resources away from normal cells and organs
- Autoimmune Diseases. Autoimmune means our own defence system is attacking our own organs and tissues. mAbs help to calm this aggression by blocking specific pathways in diseases like rheumatoid arthritis, lupus, and psoriasis, reducing inflammation and symptoms.
- Infectious Diseases: Some mAbs can neutralize viruses or bacteria, providing targeted treatment to patients.
Monoclonal Antibodies in Cancer Treatment

Monoclonal antibodies are bridging the existing gap in cancer treatment, providing more and more targeted options and shifting the paradigm
from:
“Let’s just poison the whole body in a calculated move that cancer might be more affected than normal organs and tissues”.
to:
“Let’s try to snipe cancer cells, minimizing impact on the rest of the body”.
There are at least four distinct groups of monoclonal antibodies developed to target cancer cells:
- Negative checkpoint inhibitors;
- Inducers of cancer programmatic death (apoptosis);
- Cancer growth blockers;
- Inhibitors of building new blood vessels (angiogenesis).
Group 1. Negative Checkpoint Inhibitors
Checkpoint inhibitors are a type of monoclonal antibody that helps the immune system recognize and attack cancer cells.
Remember the math: “Minus and minus gives plus“. This is the exact logic of Negative Checkpoint inhibitors:
- Negative checkpoints, when activated, put the brakes on the immune response.
- Antibodies target Negative checkpoints, enhancing anti-cancer immunity.
Here’s a simplified version:
This article gives a brief overview of this large group of biologics. For more detailed information, check out another Pharmapoet article.
Program Death Receptor (PD-1) inhibitors:
- Nivolumab (Opdivo) works by blocking the PD-1 protein on T cells. This action boosts your immune response against cancer.
- Pembrolizumab (Keytruda) also targets PD-1, enhancing the ability of your immune system to attack tumors.
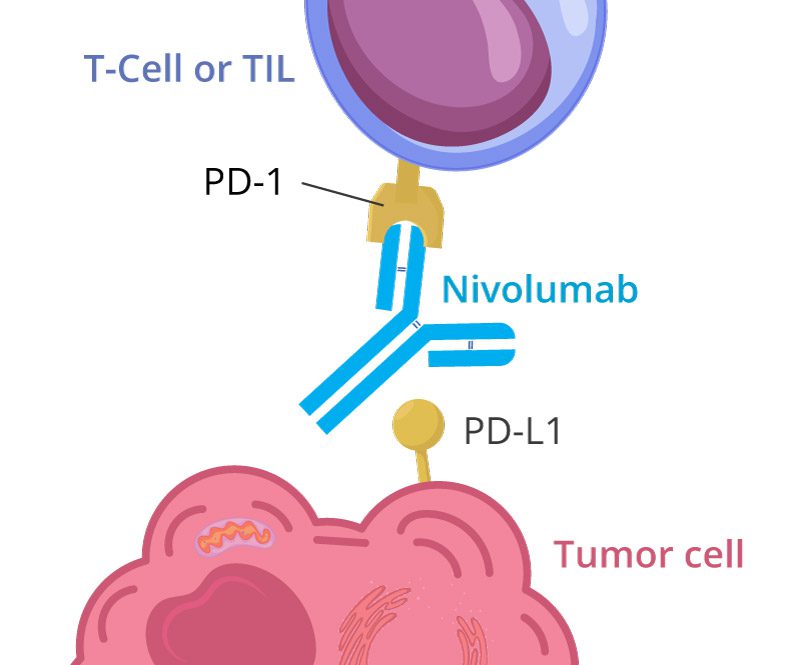
Program Death Ligand (PD-L1) inhibitors:
- Atezolizumab (Tecentriq and Tecentriq Hybreza)
- Avelumab (Bavencio)
- Durvalumab (Imfinzi)
These therapies can be used in various cancers, including melanoma, lung cancer, and kidney cancer. With checkpoint inhibitors, many patients have experienced better outcomes and longer survival rates.
Group 2. Inductors of Cancer Cell Apoptosis
Monoclonal antibodies can also induce apoptosis, which is a process that leads to programmed cell death in cancer cells.
Examples include:
- Rituximab targets CD20 on B-cell non-Hodgkin lymphoma. This binding triggers apoptosis in cancerous B cells.
- Trastuzumab (Herceptin) focuses on HER2-positive breast cancer, leading to targeted destruction of those cells.
This approach can make treatments more effective and reduce the chance of the cancer spreading. Monoclonal antibodies continue to offer new options for patients fighting cancer, significantly impacting survival and quality of life.
Group 3. Anti-growth factors
Tumors are extremely good in promoting their own growth and development, stealing and re-directing vital nutrients to the cancer cells. They have an array of chemicals in their toolbox, which scientists call tumor-growth factors, with some of them
- Epidermal Growth Factor Receptor (EGFR)
- Human Epidermal Growth Factor Receptor 2 (HER2).
Monoclonal antibodies, such as Cetuximab and Trastuzumab are developed to block these growth factors and slow the tumor growth and development.
Group 4. Angiogenesis Blockers
Tumors require a blood supply to grow beyond a certain size. They release growth factors like VEGF (vascular endothelial growth factor), which stimulate the growth of new blood vessels (angiogenesis) towards the tumor.
Antibodies that target these angiogenic factors or their receptors can inhibit tumor growth.
▪ Avastin (bevacizumab) is a humanized IgG1 antibody against VEGF-A that inhibits angiogenesis. It was first approved for metastatic colon cancer in combination with chemotherapy and later for other cancers like lung, renal, ovarian, and glioblastoma.
▪ Cyramza (ramucirumab) is a fully human IgG1 antibody that binds to the extracellular domain of VEGFR2, blocking the binding of its ligands VEGF-A, -C, and -D.
Monoclonal Antibodies in Immune Response
The immune system works around the clock to remove harmful or unwanted agents from your body.
Mistakes can happen, and sometimes, times immune system attacks your own organs and tissues, producing billions of antibodies to kill your cells. These types of mistakes can happen in the form of sporadic “autoimmune reaction”. Sometimes a person can get chronically ill, and a more sustained immune response develops against normal tissues and organs, then we call it an “autoimmune disease“. The prime example is rheumatoid arthritis or lupus.
Interleukin Inhibition
Interleukins are proteins that help regulate immune responses. Some monoclonal antibodies work by inhibiting specific interleukins. For example, anti-IL-6 antibodies can block the action of interleukin-6, which is involved in inflammation.
By blocking these interleukins, monoclonal antibodies can reduce excessive immune responses. This approach is useful in treating autoimmune diseases, where the body attacks its own tissues. Reducing inflammation can alleviate symptoms and improve your quality of life.
Advantages and Challenges
Monoclonal antibodies offer significant benefits in treating various diseases, especially in immunotherapy. However, there are also challenges related to resistance and side effects that can impact their effectiveness.
Efficacy and Precision
Monoclonal antibodies are designed to target specific antigens on cells. This precision allows them to attack diseased cells while sparing healthy ones.
Key Benefits:
- High specificity: They bind only to their target, reducing damage to normal tissues.
- Strong efficacy: Many treatments show promising results in cancer and autoimmune diseases.
- Combination therapy: They often work well with other treatments, enhancing overall effectiveness.
This targeted approach can lead to improved outcomes for patients compared to traditional therapies.
Resistance and Side Effects
Despite their benefits, monoclonal antibodies face challenges such as resistance and side effects. Some tumors can adapt, making treatments less effective over time.
Common Issues:
- Resistance: Cancer cells may change their surface markers, making them unrecognizable to the antibody.
- Side effects: Common side effects include fever, chills, and allergic reactions. Some patients experience more serious issues like infusion-related reactions.
These challenges can affect treatment plans and require careful monitoring by healthcare providers.
Future Directions in mAb Therapy
More than 200 therapeutic mAbs are available for clinicians to date. With more than 1400 candidates being investigated, the future is looking bright for mAbs.
Innovations in Design
New designs of monoclonal antibodies are changing treatment approaches. Scientists are exploring biologics that better target specific cells or pathways. This precision can reduce side effects while improving efficacy.
Receptor constructs are another innovation. These are designed to activate or block specific pathways in your body. This targeted action can lead to more effective therapies against cancers or autoimmune diseases. For example, modified antibodies with dual functions can attack cancer cells while also stimulating the immune system.
These innovations will likely increase the range of diseases treatable with mAbs. They help create more personalized medicine tailored to individual patients’ needs.
Combination Therapies
Combination therapies are gaining attention in mAb treatment. By pairing monoclonal antibodies with other treatments, such as chemotherapy or immunotherapy, you can enhance their effectiveness.
For instance, using mAb therapy alongside traditional treatments may improve patient outcomes. This approach can help overcome resistance that some cancers develop. The right combinations can also help target multiple pathways at once, increasing the chance of successful treatment.
Furthermore, combining mAbs with other biologics can lead to synergistic effects. These combinations can be particularly beneficial in treating complex diseases, where multiple mechanisms contribute to disease progression. This strategy can pave the way for more robust treatment plans.
Key Takeaways
- Your immune system makes antibodies to fight bugs.
- In 1975, scientists designed a “hybridoma”, the first “factory for antibodies”. Then researchers found a way to streamline the production, purify, and stabilize it, and the first “therapeutic monoclonal antibodies” started seeing the market.
- Antibodies have a single specific target, usually it is a protein, that was recognised by the immune system as a foe. Sometimes your immune system might attack your own organs and tissues, which we call “autoimmune reaction” or if it is a chronic condition, it is called “autoimmune disease”.
- First, Monoclonal antibodies were designed to calm “autoimmune reactions“, such as “Graft versus host disease”, when the immune system attacks a transplanted organ, seeing it as an enemy. It was muromonab-CD3.
- The next generation of mAbs has expanded their application to cancers and chronic autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, psoriasis, and a dozen others.
- The area of therapeutic mAbs is developing rapidly, bringing more tools and opportunities for doctors and patients.